Chromatography Polarity Of Solvents . the solvent used for chromatography will be selected based on the polarity of the substances in the. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Moderately polar compounds have a greater attraction to the mobile. (13.3) δ m = ∑ j δ j φ j where δ j. or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first and the least polar. a number between 0 and 1 represents the rf value and is affected by several factors, including the type of stationary phase, the polarity of the solvent,. in rplc, the polarity of a mixture of solvents is usually estimated as follows: an increase in solvent polarity increases \(r_f\) values for two reasons: The more polar the solvent, the greater its. if the mobile phase is a solvent, it is called the eluting solvent.
from www.chegg.com
the solvent used for chromatography will be selected based on the polarity of the substances in the. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. in rplc, the polarity of a mixture of solvents is usually estimated as follows: (13.3) δ m = ∑ j δ j φ j where δ j. or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first and the least polar. The more polar the solvent, the greater its. a number between 0 and 1 represents the rf value and is affected by several factors, including the type of stationary phase, the polarity of the solvent,. an increase in solvent polarity increases \(r_f\) values for two reasons: Moderately polar compounds have a greater attraction to the mobile. if the mobile phase is a solvent, it is called the eluting solvent.
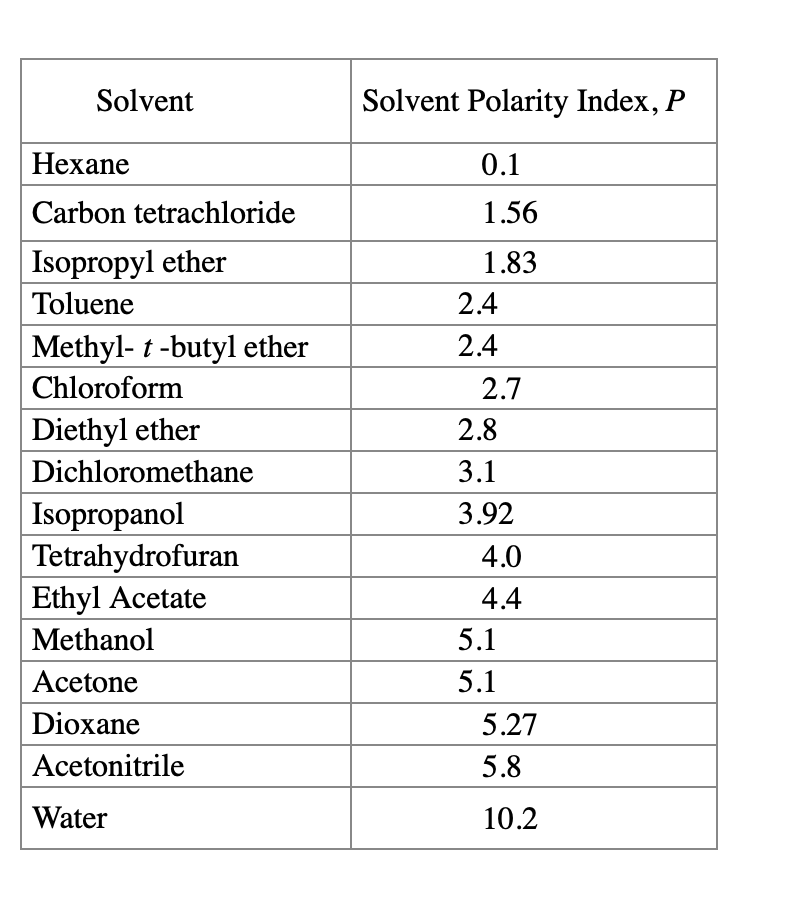
Solved Determine the solvent polarity index for each HPLC
Chromatography Polarity Of Solvents (13.3) δ m = ∑ j δ j φ j where δ j. (13.3) δ m = ∑ j δ j φ j where δ j. or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first and the least polar. Moderately polar compounds have a greater attraction to the mobile. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. an increase in solvent polarity increases \(r_f\) values for two reasons: the solvent used for chromatography will be selected based on the polarity of the substances in the. if the mobile phase is a solvent, it is called the eluting solvent. in rplc, the polarity of a mixture of solvents is usually estimated as follows: a number between 0 and 1 represents the rf value and is affected by several factors, including the type of stationary phase, the polarity of the solvent,. The more polar the solvent, the greater its.
From techblog.ctgclean.com
Chemistry Solvent Characteristics CTG Clean Chromatography Polarity Of Solvents (13.3) δ m = ∑ j δ j φ j where δ j. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. if the mobile phase is a solvent, it is called the eluting solvent. The more polar the solvent, the greater its. in rplc, the polarity of a mixture of solvents is. Chromatography Polarity Of Solvents.
From dxoosotti.blob.core.windows.net
Tlc Increase Polarity Of Solvent at Eugene Croce blog Chromatography Polarity Of Solvents if the mobile phase is a solvent, it is called the eluting solvent. a number between 0 and 1 represents the rf value and is affected by several factors, including the type of stationary phase, the polarity of the solvent,. Moderately polar compounds have a greater attraction to the mobile. Chromatography is regarded as one of the most. Chromatography Polarity Of Solvents.
From www.youtube.com
Selection of solvent in chromatography column chromatography chromatography experiment Chromatography Polarity Of Solvents Chromatography is regarded as one of the most significant bioanalytical techniques used in different. in rplc, the polarity of a mixture of solvents is usually estimated as follows: The more polar the solvent, the greater its. an increase in solvent polarity increases \(r_f\) values for two reasons: Moderately polar compounds have a greater attraction to the mobile. . Chromatography Polarity Of Solvents.
From www.chegg.com
Solved Determine the solvent polarity index for each HPLC Chromatography Polarity Of Solvents if the mobile phase is a solvent, it is called the eluting solvent. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Moderately polar compounds have a greater attraction to the mobile. or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first. Chromatography Polarity Of Solvents.
From stock-picture.com
thin layer chromatography image Big Image Stock Chromatography Polarity Of Solvents an increase in solvent polarity increases \(r_f\) values for two reasons: a number between 0 and 1 represents the rf value and is affected by several factors, including the type of stationary phase, the polarity of the solvent,. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. The more polar the solvent, the. Chromatography Polarity Of Solvents.
From bceweb.org
Solvent Polarity Chart A Visual Reference of Charts Chart Master Chromatography Polarity Of Solvents Chromatography is regarded as one of the most significant bioanalytical techniques used in different. in rplc, the polarity of a mixture of solvents is usually estimated as follows: the solvent used for chromatography will be selected based on the polarity of the substances in the. or, if the experiment requires a solvent polarity gradient, the gradient must. Chromatography Polarity Of Solvents.
From bceweb.org
Solvent Polarity Chart A Visual Reference of Charts Chart Master Chromatography Polarity Of Solvents an increase in solvent polarity increases \(r_f\) values for two reasons: or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first and the least polar. (13.3) δ m = ∑ j δ j φ j where δ j. in rplc, the polarity of a mixture of. Chromatography Polarity Of Solvents.
From alexmistry.z13.web.core.windows.net
Polarity Of Organic Solvents Chart Chromatography Polarity Of Solvents in rplc, the polarity of a mixture of solvents is usually estimated as follows: (13.3) δ m = ∑ j δ j φ j where δ j. Moderately polar compounds have a greater attraction to the mobile. or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first. Chromatography Polarity Of Solvents.
From shawhan-z-bio.com
Research on the rapid selection of suitable solvent system for HSCCC based on the HPLC polarity Chromatography Polarity Of Solvents in rplc, the polarity of a mixture of solvents is usually estimated as follows: if the mobile phase is a solvent, it is called the eluting solvent. The more polar the solvent, the greater its. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. a number between 0 and 1 represents the. Chromatography Polarity Of Solvents.
From dokumen.tips
(PDF) Column Selection for Size Exclusion Chromatography (SEC) · High Polarity of Solvent Low 42 Chromatography Polarity Of Solvents (13.3) δ m = ∑ j δ j φ j where δ j. the solvent used for chromatography will be selected based on the polarity of the substances in the. if the mobile phase is a solvent, it is called the eluting solvent. Moderately polar compounds have a greater attraction to the mobile. a number between 0. Chromatography Polarity Of Solvents.
From travistra.blogspot.com
COLUMN Chromatography Polarity Of Solvents in rplc, the polarity of a mixture of solvents is usually estimated as follows: the solvent used for chromatography will be selected based on the polarity of the substances in the. a number between 0 and 1 represents the rf value and is affected by several factors, including the type of stationary phase, the polarity of the. Chromatography Polarity Of Solvents.
From mavink.com
Gc Column Polarity Chart Chromatography Polarity Of Solvents if the mobile phase is a solvent, it is called the eluting solvent. in rplc, the polarity of a mixture of solvents is usually estimated as follows: or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first and the least polar. the solvent used for. Chromatography Polarity Of Solvents.
From lolabooth.z13.web.core.windows.net
Polarity Of Solvents Chart Chromatography Polarity Of Solvents Moderately polar compounds have a greater attraction to the mobile. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first and the least polar. an increase in solvent polarity increases \(r_f\) values for. Chromatography Polarity Of Solvents.
From dxoosotti.blob.core.windows.net
Tlc Increase Polarity Of Solvent at Eugene Croce blog Chromatography Polarity Of Solvents The more polar the solvent, the greater its. Moderately polar compounds have a greater attraction to the mobile. or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first and the least polar. the solvent used for chromatography will be selected based on the polarity of the substances. Chromatography Polarity Of Solvents.
From mavink.com
Solvent Polarity Chart For Tlc Chromatography Polarity Of Solvents in rplc, the polarity of a mixture of solvents is usually estimated as follows: the solvent used for chromatography will be selected based on the polarity of the substances in the. (13.3) δ m = ∑ j δ j φ j where δ j. a number between 0 and 1 represents the rf value and is affected. Chromatography Polarity Of Solvents.
From www.researchgate.net
Comparison of the Polarity Index of Organic Solvents Download Scientific Diagram Chromatography Polarity Of Solvents an increase in solvent polarity increases \(r_f\) values for two reasons: Chromatography is regarded as one of the most significant bioanalytical techniques used in different. a number between 0 and 1 represents the rf value and is affected by several factors, including the type of stationary phase, the polarity of the solvent,. The more polar the solvent, the. Chromatography Polarity Of Solvents.
From www.waters.com
Hydrophilic Interaction Chromatography Using Silica Columns for the Retention of Polar Analytes Chromatography Polarity Of Solvents Moderately polar compounds have a greater attraction to the mobile. if the mobile phase is a solvent, it is called the eluting solvent. in rplc, the polarity of a mixture of solvents is usually estimated as follows: The more polar the solvent, the greater its. the solvent used for chromatography will be selected based on the polarity. Chromatography Polarity Of Solvents.
From www.researchgate.net
Solvent polarity and eluotropic strength (ɛ°) Download Scientific Diagram Chromatography Polarity Of Solvents a number between 0 and 1 represents the rf value and is affected by several factors, including the type of stationary phase, the polarity of the solvent,. or, if the experiment requires a solvent polarity gradient, the gradient must be carried out with the most polar solvent first and the least polar. the solvent used for chromatography. Chromatography Polarity Of Solvents.